Apr 11
19
Combustion is the breakdown of an organic material, in combination with oxygen to form carbon dioxide and water and energy. This energy is heat and light, which used in everything from campfires to internal combustion engines.
Combustion of an organic material is simply the rapid acceleration of the natural decomposition process. If dead wood is left on the forest floor and in the presence of natural heat humidity and microbial action it will break down into simpler matter. How quickly this occurs is dependant on temperature, oxygen, moisture etc.
The higher the temperature, the quicker the decomposition. If high heat is applied to the log, from a match or flame from other sources for example, the organic conversion will be rapidly accelerated.
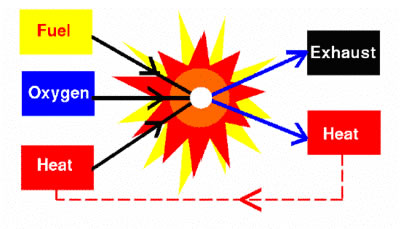
Combustion is defined as the burning of a fuel and oxidant to produce heat and/or work. It is the major energy release mechanism in the Earth and key to humankind’s existence.
Combustion includes thermal, hydrodynamic, and chemical processes. It starts with the mixing of fuel and oxidant, and sometimes in the presence of other catalysts. The fuel can be gaseous, liquid, or solid and the mixture may be ignited with a heat source.
When ignited, chemical reactions of fuel and oxidant take place and the heat release from the reaction creates a self-sustained process. The combustion products include heat, light, gases, chemical species, pollutants, mechanical work, and plasma.
Sometimes, a low-grade fuel, e.g., coal, biomass, or coke, can be partially burned to produce higher-grade fuel, e.g., methane. The partial burning process is called gasification.

 EXECUTIVE DOWNLOAD
EXECUTIVE DOWNLOAD